Featured topic
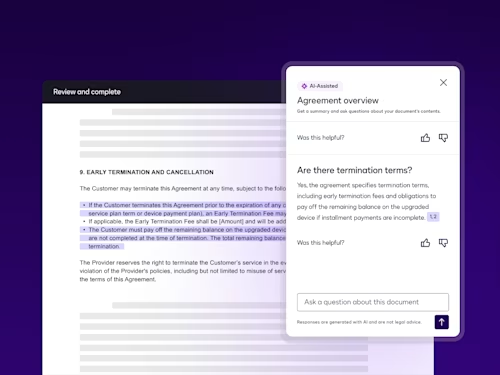
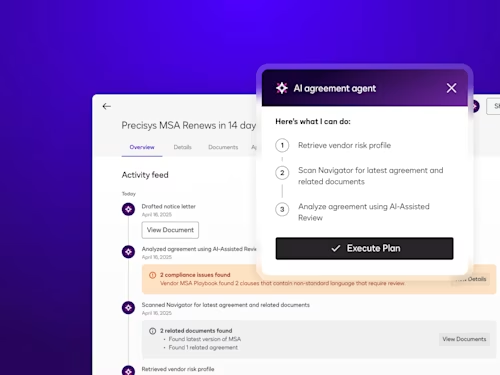
Intelligent Agreement Management
See how Intelligent Agreement Management helps organizations to create, commit to, and manage agreements through a single, streamlined workflow.
Popular posts
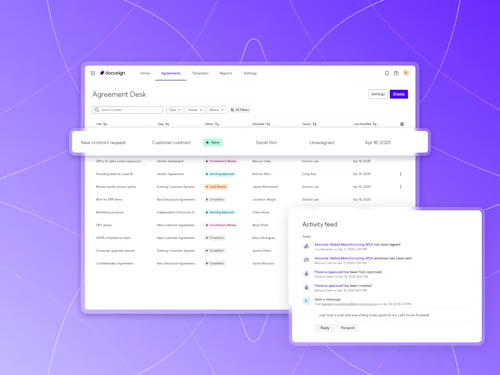
Digitize and Organize Your Agreements in a Central Repository
With Docusign IAM, all existing eSignature agreements are automatically stored in Navigator.

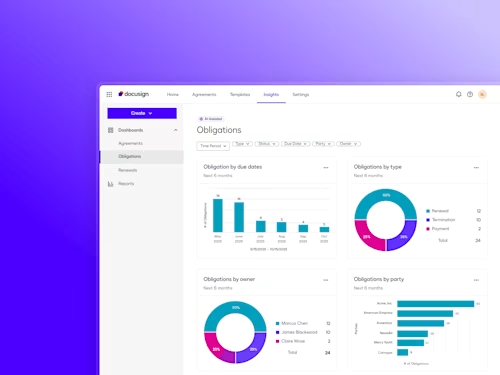
Docusign IAM is the agreement platform your business needs