Docusign IAM | Life Sciences Modules for 21 CFR Part 11
Spend time on patients, not paperwork
Improve the patient, physician and employee experience

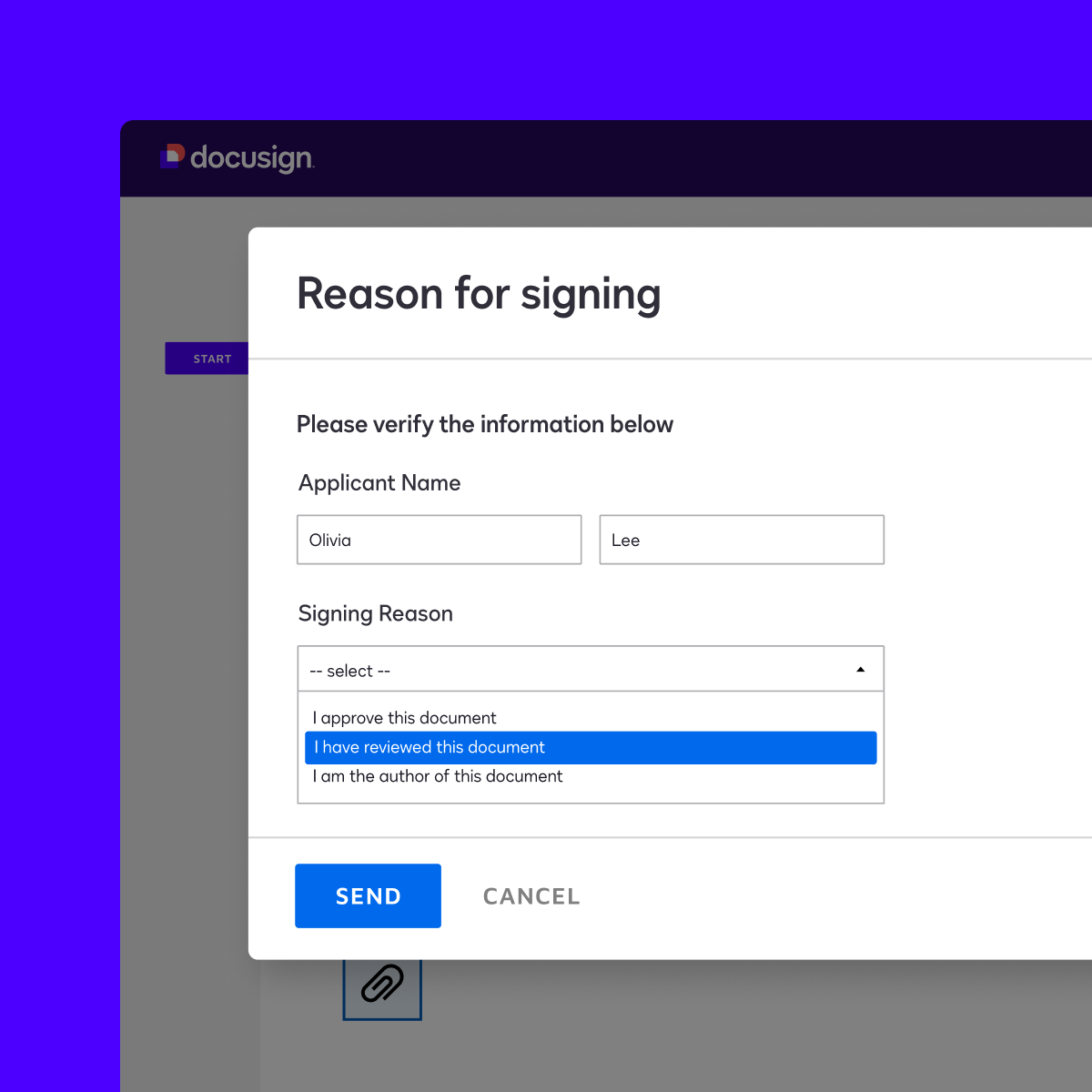
Simplify compliance
Docusign offers modules to support your compliance with the electronic signature practices set forth in the U.S. Food and Drug Administration’s 21 CFR Part 11. Our core Part 11 module includes Part 11-specific eSignature functionality for authentication, reason for signature and signature manifestation. These capabilities help you comply with regulations while using eSignature to make executing agreements faster, more cost-efficient and more convenient for everyone involved.
- Account-wide controls
Apply module settings to all envelopes sent from your account to enable consistent compliance.
- Simplify compliance validation
Receive automated reports regarding Part 11 functionality compliance.
- Authenticate two ways
Require two-factor authentication to access and sign documents.
- Know who did what
View a detailed audit trail and Certificate of Completion for each transaction.
FAQ
Life sciences organisations use Docusign to reduce time to market, simplify global collaboration, and improve the patient, physician, and employee experience.
See the support page for the Validator.
Docusign IAM is the agreement platform your business needs
